Thermodynamics is essentially the study of energy transfers to objects and the changes in the internal energy of the object or the work done by the object as a result.
Heat is energy that is transferred due to temperature differences. If heat is transferred to an object it can either increase its internal energy or it can do work. Energy transfer very often involves heating the surroundings. In most cases heat transfer makes energy spread out.
The laws of thermodynamics were established in the 19th century and they are very important because no experiments have ever been able to disprove them. To understand the laws we must consider them in atomic terms. Although the behaviour of individual atoms is random in nature the behaviour of very large numbers of atoms is very predictable.
The internal energy is the energy stored by an object as the potential and kinetic energy of its molecules.
Suppose an object gains energy due to heat transfer and work done on it. Then the internal energy must increase by an amount equal to the heat transfer + the work done. This states the principle of conservation of energy and is also the First Law Of Thermodynamics
![]()
where DQ is the heat supplied to the system, DU is the change in internal energy and DW is the work done by the system.
Every object contains a certain amount of internal energy, which is contained within the random motion of its molecules. The total amount of internal energy depends on its
temperature
composition
mass
physical state
However it is only the temperature of the body, which determines whether energy will be transferred from it to another body with which it is in contact or vice versa. A large block of ice has far more internal energy than a cup of hot water yet when the water is poured on the ice some of it melts and the water becomes cooler, which signifies that some energy has passed from the water to the ice.
Heat is internal energy in transit.
Heat is a quantity that causes an increase in the temperature of a body to which it is added, and a decrease in the temperature of a body from which it is taken away, provided that the body does not change state during the process.
Internal energy consists of both PE and KE of a body. The PE is due to the vibration of the molecules and the KE is due to the translational, rotational and vibrational motion of the molecules. The motion of the molecules is random and at any instant in time the atoms in a body will have a range of speeds due to collisions etc. Since KE a speed2 then atoms have a range of energies at any instant in time
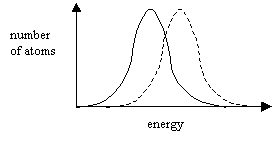
Normal
Distribution – the graph shows that a small number of atoms have a
very small energy and a small number of atoms have a very high energy.
When the temperature is increased the whole graph moves to the right
Links to more information: