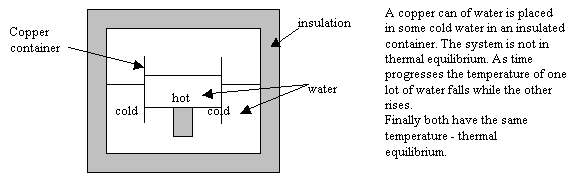
 |
All energy exchange appears to stop after a time when hot and cold bodies are brought into contact. The bodies are now said to be in thermal equilibrium. We call the common property they have… temperature.
Any
two objects in thermal contact are in thermal equilibrium if there is no overall
heat transfer between them.
ie. If something at high temperature is placed into contact with something at low temperature then heat energy will flow from high temperature to low temperature until both bodies have the same temperature.
 |
An everyday
example : The baby and the bathwater

A baby's bath has to be the right temperature. Too cold and heat is transferred away from the baby, too hot and heat is transferred to the baby ! Ideally the water should be at the same temperature as the baby.
To ensure that the bathwater is just right a skilled parent can hold the baby just above the water while simultaneously dipping an elbow into the water. If the elbow and the water are in thermal equilibrium, then the baby and the parent are in thermal equilibrium and hence the baby and the bathwater are in equilibrium and the water will be safe for the baby.
The parent has used the zeroth law of thermodynamics, which states
If
body A is in thermal equilibrium with body B, and body B is in thermal
equilibrium with body C, then body A must be in thermal equilibrium with body C.