Work done on an object can raise its temperature. (This can
be used to light fires by rubbing sticks together). These notes will explain the
experimental approach to linking heat energy with temperature change.
In 1798 Count Rumford performed the first conclusive
experiment which demonstrated that heat energy appeared when work was done on an
object. He was Bavaria's Minister of War at the time. One of his tasks was to
supervise the manufacture of ammunition and cannon. The latter were made by
boring large holes into cast brass. using horses as the energy source to drive
the boring tool. Rumford observed that copious quantities of heat energy were
produced in the process. For example, he surrounded one cannon with water and
found that a single horse turning a blunt boring tool could do enough work to
raise 12 kg of water from 0° C to 100°
C in 2 hours, without apparently changing the brass. He concluded from his
experiments that the amount of heat energy produced was proportional to the work
done by the horse
Between
1843 and 1850 James Joule performed a number of experiments to find out how much
work had to be done on a fixed mass of water to raise its temperature by 1°C.
The diagram illustrates one of his pieces of apparatus, the work done by the
falling weights was converted to heat energy in the water by churning it with
paddles. He found that about 4200 J of work, regardless of how it was done, was
sufficient to raise the temperature of 1 kg
of water by 1° C.
From
the earliest experiments it was obvious that the heat energy DQ
generated by the work was linked to the temperature change DT by an equation of this form,
![]()
DQ is
the heat energy given to an object (J)
m
is the mass of the object (kg)
c
is the specific heat capacity of the object (Jkg-1K-1)
DT is the
temperature rise of the object (K)
The value of the specific heat capacity
varies from one substance to another. It is the heat energy required to raise
the temperature of 1kg of a substance by 1K. It is not a constant in fact, its
value does vary with temperature but the variation is sufficiently small above
temperatures of about 400K for its value to be precise enough for most
applications.
To accurately measure the specific heat capacity of a substance several quantities need to be known.
· amount of heat energy
· temperature rise
· mass of substance being heated
The most convenient way of calculating the
heat energy input is to use an electrical heater and to measure the voltage and
current, the power of the heater being given by
![]() .
.
The initial temperature T of the object is determined using a thermometer, the heater is then switched on for a time interval t. After the heater is switched off the final temperature T + DT is noted.
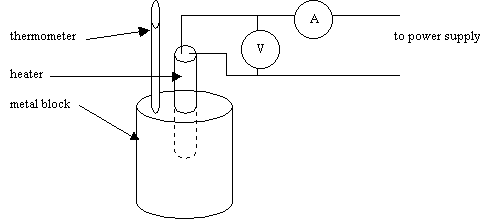
The metal block will lose heat energy to its surroundings as soon as its temperature is raised above the temperature of the surroundings.
What
can be done to minimise heat loss by
a)
conduction
b)
convection
What
will be the effect of energy losses be on the final temperature reached ? Should
we expect an under or overestimate of c ?
If we record the cooling of the block after switching off the heater we can estimate the heat energy lost from the block during heating by measuring the temperature drop dT in a small time dt halfway down the cooling curve. The actual heat energy supplied can then be used to calculate a more accurate value of c.
|
mass of aluminium cylinder |
m |
|
|
initial temperature |
T |
|
|
final temperature |
T + DT |
|
|
heater voltage |
V |
|
|
heater current |
I |
|
|
heating time |
t |
|
Calculate the heat energy supplied to the block
............................................................................................................................................................................
............................................................................................................................................................................
............................................................................................................................................................................
............................................................................................................................................................................
Calculate a value for the specific heat capacity of aluminium
............................................................................................................................................................................
............................................................................................................................................................................
............................................................................................................................................................................
............................................................................................................................................................................
Estimate the heat energy lost from the block during heating by measuring the temperature drop of the block for the same time period that the heater was switched on for about ten minutes after you have switched off the heater.
............................................................................................................................................................................
............................................................................................................................................................................
............................................................................................................................................................................
............................................................................................................................................................................
Finally calculate the actual heat energy delivered to the block during heating and recalculate a more accurate value for the specific heat capacity of aluminium.
............................................................................................................................................................................
............................................................................................................................................................................
............................................................................................................................................................................
............................................................................................................................................................................
There is one major source of error in this experiment to measure the specific heat capacity of aluminium, which has not been considered. What is this source of error and how should it be minimised ?
............................................................................................................................................................................
............................................................................................................................................................................
............................................................................................................................................................................
............................................................................................................................................................................